Nano-flow LC (nano-LC) is an important tool in proteomics research and in the measurement of low level biomarkers. To avoid the common problem of emitter clogging,only the highest purity solvents and reagents should be used.
Connecting a liquid chromatography (LC) system to a mass spectrometer with an electrospray ionization (ESI) source, LC/MS or LC/MS/MS (MS/MS for tandem mass spectrometry), offers today’s laboratories superior sensitivity and dynamic range over other detectors, and provides far more molecularly-specific information. However, minute sample quantities, complex matrices and very low concentrations (pg/mL) make analysis of endogenous biomolecules found in vivo a challenge. An excellent solution is provided by nanoflow LC (nano-LC) coupled to nano-electrospray ionization (nanospray), which typically utilizes 75 µm ID columns. Nano-LC requires minimal sample loading and results in more concentrated peaks. The very low flow rate (nL/min range) is optimal for nanospray sources. Nanospray ionization increases ionization efficiency, leading to better sensitivity.
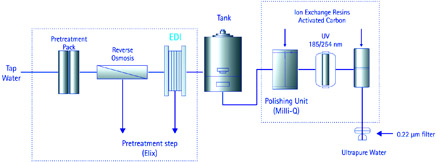
Nano-LC/MS emitters/ sprayers have tips with inner diameters of ~1-30 µm that are prone to clogging. It is therefore important to choose not only the most robust emitters, but also the highest purity solvents and reagents. This work uses the separation of neuropeptides by nano-LC/MS to demonstrate that using freshly produced ultrapure water as a solvent component in nano-LC/MS does not clog emitters.
Experiments
The nano-LC/MS system consisted of a Waters nanoACQUITY UPLC system with a Quattro Premier XE MS/MS equipped with a nanospray source (Waters, Milford, MA) operated at a flow of 900 nL/min. A Waters Atlantis dC18 (75 µm x 100 mm, 3 µm) analytical column; PicoTip SilicaTip, 360 µm OD, 10 µm tip ID emitters (New Objective, Woburn, MA); and a methylated monolithic silica MonoSpray, 360 µm OD, 50 µm tip ID (GL Sciences, Torrance, CA) were used.
 The mobile phase in the gradient elution was composed of (A) ultrapure water from a Milli-Q Gradient system with an A10 monitor (EMD Millipore, Billerica, MA) containing 0.2% acetic acid (BDH Aristar Ultra acetic acid from VWR, West Chester, PA) and 1% acetonitrile (B&J acetonitrile UV, Honeywell Burdick & Jackson, Morristown, NJ), and (B) 100 % acetonitrile. The mobile phase in the gradient elution was composed of (A) ultrapure water from a Milli-Q Gradient system with an A10 monitor (EMD Millipore, Billerica, MA) containing 0.2% acetic acid (BDH Aristar Ultra acetic acid from VWR, West Chester, PA) and 1% acetonitrile (B&J acetonitrile UV, Honeywell Burdick & Jackson, Morristown, NJ), and (B) 100 % acetonitrile.
Angiotensin, leu-enkephalin and met-enkephalin neuropeptide biomarker standards were used, with different concentrations injected to monitor changes in retention times, peak areas, calibration curves, and the physical state of the emitter. Injection volume was 1 µL. An Olympus BH-2 microscope was used to take photomicrographs of emitters.
Results
Emitter clogging is the most common problem associated with nano-LC/MS experiments. In this work, the aqueous mobile phase was made from freshly delivered ultrapure water obtained from a water purification system (Figure 1) employing a combination of purification technologies: reverse osmosis, electrodeionization (Elix module), high grade ion exchange resins, synthetic activated carbon, and UV photooxidation.
This combination of technologies results in ultrapure water with a consistent quality: ion-free, and with the lowest possible levels of organics (thus avoiding accumulation in reversed-phase columns that could later elute as extraneous peaks in the chromatogram). When water is delivered using a 0.22 µm membrane final filter, this filter also retains bacteria and particulates (> 0.22 µm) that could clog emitters.
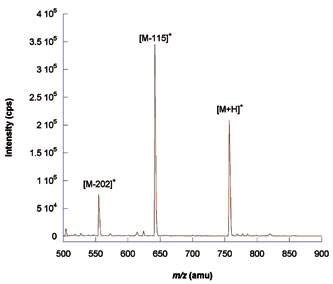
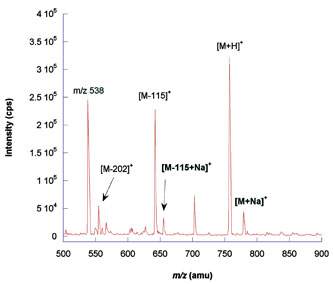
Keeping ionic contamination of solvents to a minimum is important. Ions such as Na+ and K+ form adducts with peptides, complicating data analysis and reducing sensitivity. Figure 2A shows the difference in the mass spectrum of a bradykinin standard dissolved in fresh ultrapure water and directly infused onto a mass spectrometer, and when it is dissolved in ultrapure water spiked with sodium ions (Figure 2B).
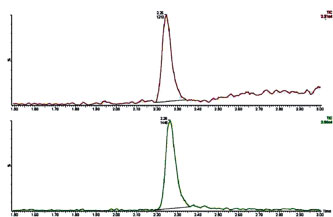
Experiments were also carried out to verify that fresh ultrapure water does not clog emitters. Figure 3 shows mass chromatograms of leu-enkephalin where fast LC conditions were used for over one hundred back-to-back injections, with fresh ultrapure water making up the aqueous mobile phase. Retention times and peak areas remained essentially unchanged between early (#5) and late (#111) injections. Comparisons of the mass chromatograms of met-enkephalin and angiotensin (not shown) also remained constant. The excellent run-to-run reproducibility indicates that there was no clogging of the emitter.
Comparison of standard calibration plots after prolonged use of an emitter would give an indication of the condition of the emitter.
To further inspect emitter clogging, the physical appearance of emitters used for one month and unused emitters were examined under a light microscope. Photomicrographs did not reveal any clogging.
The flow rate used in this work (900 nL/min) is high for nano-scale work, yet the emitters tested did not show signs of deterioration due to clogging. Thus, using freshly produced ultrapure water delivered out of a 0.22 µm membrane filter at the water purification system’s point of use is strongly recommended for nano-LC/MS analyses.
Conclusions
This work demonstrated that emitter clogging does not take place when fresh ultrapure water filtered through a 0.22 µm point-of-use filter is used in eluent preparation for nano-LC/MS. Retention times, peak areas, and calibration plots of the neuropeptides were unchanged after extended use of the emitters. Photomicrographs of a used emitter did not reveal any evidence of debris or clogging. Fresh ultrapure water has consistent quality, and contains minimal levels of organic and ionic contaminants, all of which are crucial in robust and sensitive nano-LC/MS analyses of biological samples.
(Maricar Tarun is EMD Millipore, Billerica, MA, USA, Stéphane Mabic is working with Merck Millipore, St Quentin-en-Yvelines, France and Schwalbach, Germany and David P. Budac and Mark J. Hayward are working with Lundbeck Research, Paramus, NJ, USA) |