This article provides an overview of current drug shortages in the United States as on November 5, 2011. IMS report finds cancer drugs and generic injectables are scarcest, calls for early warning system to prevent shortages from disrupting patient care. Shortages are worst among a handful of 75 drugs, including biologicals, vitamins, antibiotics, antiemetics, antihypertensive agents, narcotic analgesics and CNS drugs. Most of the drugs are generic intravenous or injectable drugs given to patients in hospitals. Manufacturers voluntarily report shortages to the US FDA. There are several reasons for current drug shortages. A major reason for these shortages is quality/manufacturing issues and growing demand for these drug products. Drug shortage is a long-standing problem in the US and in many countries worldwide. There is a great need to develop a plan addressing this critical issue.
More than 200 pharmaceutical drugs are on the current shortage list in the United States. If you talk with patients, pharmacists or physicians in America, you would realize shortage of many drug products. As per the reports by the US Food and Drug Administration (US FDA), which provides list of current drug shortages, it is clear that United States is facing big drug shortage this year. US faces a critical shortage of many chemotherapy drugs, forcing some patients to delay their treatments or switch medications. And it is not just cancer drugs; there are more than 200 drugs on the US FDA’s shortage list – including biologicals, vitamins, antibiotics, antiemetics, antihypertensives, narcotic analgesics and CNS drugs. Most of the drugs on the list are generic intravenous or injectable drugs given to patients in hospitals.
Drug shortages vary periodically
The number of drugs on the shortage list has nearly tripled in the last six years. In 2010, there were 178 drug shortages reported to the US FDA, 132 of which involved injectable products. In 2011, the country continued to see an increasing number of shortages, reaching to 246 drugs, especially those involving injectable drugs. These shortages have involved cancer drugs, anesthetics used for patients undergoing surgery, as well as drugs needed for emergency medicine, and electrolytes needed for patients on IV feeding. Such shortage ultimately affects patient safety and quality of care.
There are other several reasons for current drug shortages in the US. A major reason for these shortages is quality or manufacturing issues and growing demand for products. Constrains are noticed with active pharmaceutical ingredients (API), which are supplied by bulk drug companies. However, there have been other reasons such as production delays at the manufacturer and delay at companies receiving raw materials and components from suppliers. Aside from changes in clinical practices, product discontinuations and product recalls are temporarily contributing to shortages because US FDA can not require a firm to keep making a drug it wants to discontinue.
Drug shortages are not a factor for price increases in the United States because of strict trade practices. However, when shortages occur, some distributors may fix higher prices than normal procurement price. Due to shortage, hospitals report having to resort to buying drugs from the third-party suppliers known for steep mark-ups on the price of drugs, according to a recent survey from the Institute for Safe Medication Practices. Moreover, drug products obtained from such channels of distribution may sometime be harmful due to inadequate storage and other quality issues.
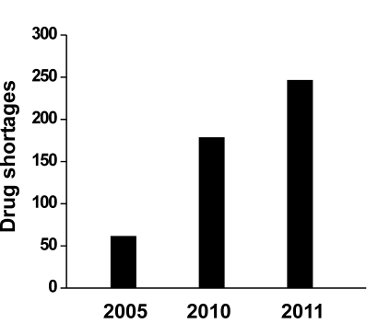
The growing drug shortages in USA
Reasons for drug shortages
The US FDA works with pharmaceutical companies and encourages them to fix production problems, while regulating the quality, but it can not enforce companies to make the drug products and it can’t regulate the price of drugs. The companies are facing constraints in dealing with raw material suppliers and adhering to quality standards. The raw material suppliers the firms use are also limited in the amount they can make due to capacity issues at their facilities.
Manufacturers voluntarily report shortages to US FDA, but there’s only so much the US FDA can do to prevent drug shortages including importation of drugs from overseas. The small number of manufacturers and limited production capacity for such drugs combined with the long lead times and complexity of the manufacturing process for injectable drugs, results in these drugs being vulnerable to shortage. When one company has a problem or discontinues, it is difficult for the remaining firms to increase production quickly and a shortage occurs.
Conclusion
Overall, drug shortage is a long-standing problem in the United States and in many countries worldwide. A real runaround is occurring with hospitals and pharmacies trying to locate these drugs, spending several hours daily trying to find suppliers of drugs they need for their patients. Continued drug shortages could lead to patient safety crisis. There is a great need to develop a plan addressing this critical issue. Despite lack of new changes in US FDA standards, it is not quite clear why so many quality problems are occurring with drugs contributing to shortage. The production process for anticancer drugs and biologicals is multifaceted and such formulations as sterile injectables is certainly complex and involves many steps contributing to manufacturing problems. Implementation of new technologies is one solution for mitigating such problems. Due to the medical necessity of these drugs, it is expected that reports of potential or actual drug shortages will continue to increase in the next months to years.
The author is Editor-in-Chief, International Journal of Pharmaceutical Sciences and Nanotechnology, and associate professor, Department of Neuroscience and Experimental Therapeutics, Texas A&M University System Health Science Center, College of Medicine, College Station, Texas, USA.
|