Synthesis, antimicrobial activity of 2-amino-4, 6-diaryl pyrimidines | | B. Ramesh, Raveendra R, Suresh V. Kulkarni, Tuesday, September 18, 2012, 08:00 Hrs [IST] |
|
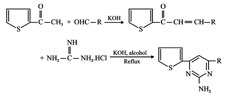
Some new pyrimidine derivatives were synthesized by reacting chalcones of 2-acetyl thiophene with guanidine hydrochloride in the presence of alcohol. The synthesized compounds were identified by spectral data and screened for antimicrobial activity. Some of these compounds showed moderate to considerable antimicrobial activity.
Compounds with pyrimidine structures are known to possess antimicrobial anti-inflammatory, cytotoxic, anti-cancer activities. In the present study some new pyrimidine derivatives (1 to 5) have been synthesized by the reaction of chalcones of 2-acetyl thiophene and guanidine hydrochloride. The structures of the various synthesized compounds are assigned on the basis of elemental analysis, IR and H NMR spectral data. These compounds were also screened for their antimicrobial activity.
Experimental
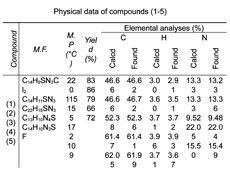
Melting points were determined on a capillary melting point apparatus and are uncorrected. H NMR spectra was recorded in the indicated solvent on Bruker WM 400 MHz spectrometer with TMS as internal standard. Infrared spectra were recorded in KBr on Perkin-Elmer AC-1 spectrophotometer. Microanalyses were performed on Carlo Erba EA-1108 element analyzer and were within the ± 0.5% of the theoretical values. Column chromatography was performed on silica gel (Merck, 60-120 mesh).
Procedure for preparation of pyrimidine derivatives (1-5)
A mixture of chalcones of 2-acetyl thiophene (0.001 mol) and guanidine hydrochloride (500 mg) in absolute ethanol (10 ml) were refluxed on a waterbath for 6 hours. The solvent was completely evaporated and the residue was poured into ice cold water. The precipitated solid was collected by filtration and crystallized from suitable solvent to give the pyrimidine derivative.
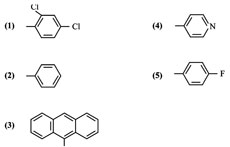
Synthesis of some new 2-amino-4, 6-diaryl pyrimidines (1-5)
Antimicrobial activity
Cup plate method using Mueller-Hinton agar medium was employed to study the preliminary antibacterial activity of (1-5) against B. pumilis, B. substilis, E. coli and P. vulgaris. The agar medium was purchased from Hi Media Laboratories Ltd., Mumbai. Preparation of nutrient broth, subculture, base layer medium, agar medium and peptone water was done as per the standard procedure. Each test compound (5 mg) was dissolved in 5 mL of dimethyl sulfoxide (1000 g/mL). Volumes of 0.05 mL and 0.1 mL of each compound were used for testing.

 Same cup plate method using PDA medium was employed to study the preliminary antifungal activity of (1-5) against A. niger and P. crysogenium. The PDA medium was purchased from Hi Media Laboratories Ltd., Mumbai. Preparation of nutrient broth, subculture, base layer medium and PDA medium was done as per the standard procedure. Each test compound (5 mg) was dissolved in 5 mL of dimethyl sulfoxide (1000 g/mL). Volumes of 0.05 mL and 0.1 mL of each compound were used for testing. Same cup plate method using PDA medium was employed to study the preliminary antifungal activity of (1-5) against A. niger and P. crysogenium. The PDA medium was purchased from Hi Media Laboratories Ltd., Mumbai. Preparation of nutrient broth, subculture, base layer medium and PDA medium was done as per the standard procedure. Each test compound (5 mg) was dissolved in 5 mL of dimethyl sulfoxide (1000 g/mL). Volumes of 0.05 mL and 0.1 mL of each compound were used for testing.
Table 1. Physical data of compounds (1-5)
The cups each of 9 mm diameter were made by scooping out medium with a sterilized cork borer in a petri dish, which was streaked with the organisms. The solutions of each test compound (0.05 and 0.1 mL) were added separately in the cups and petri dishes and were subsequently incubated. Benzyl penicillin and fluconazole were used as standard reference drugs (200 and 500 g/mL, respectively) and dimethyl sulphoxide as a control, which did not reveal any inhibition.
Results
The screening results revealed that the compounds (1-5) showed significant antibacterial activity at both 0.05 mL abd 0.1 mL concentration levels when compare with standard drug benzyl penicillin. It was found that compound 1-5 showed maximum activity. It is interesting to note that compound 1-5 is having dichloro and fluoro groups as pharmacophores respectively.

Antifungal activity
The screening results revealed that the compounds (1-5) showed significant antifungal activity at both 0.05 mL and 0.1 mL concentration levels when compared with standard drug fluconazol. In particular compounds 1-5 possessed maximum activity which may be due to the presence of dichloro and fluoro as pharmacophores respectively.
(Authors B. Ramesh and Suresh V. Kulkarni are with Department of Pharmaceutical Chemistry, Sree Siddaganga College of Pharmacy, BH. Road, Tumkur 572102, Karnataka, and Raveendra R with Dept of Pharmaceutical Chemistry, RR College of Pharmcy, Bangalore-90) |