Dr Reddy's Laboratories (DRL), the second largest Indian pharma major with consolidated net sales of Rs.15,400 crore plus, has set to overcome challenging circumstances with higher investment in research and development, focus on operational excellence and acquisitions. The company implemented structural changes for ensuring the quality after receiving US FDA warnings during 2015-16. DRL is a global generics player with 83 per cent of its sales coming from generics segment.
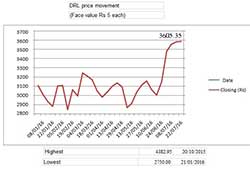 DRL scrip was under pressure after receiving warning letters from US FDA authorities during November 2015 in respect of inadequate quality control procedures at three manufacturing plants in India. The scrip, which touched to its yearly highest level at Rs.4382.95 on October 20, 2015, declined sharply below Rs.3,000 mark in January 7, 2016 and reached at its yearly lowest level at Rs.2,750 on January 21, 2016. Considering the low price, the management declared buy-back offer for Rs.3,500 per share for an aggregate amount of Rs.1,569 crore during February 2016. DRL scrip was under pressure after receiving warning letters from US FDA authorities during November 2015 in respect of inadequate quality control procedures at three manufacturing plants in India. The scrip, which touched to its yearly highest level at Rs.4382.95 on October 20, 2015, declined sharply below Rs.3,000 mark in January 7, 2016 and reached at its yearly lowest level at Rs.2,750 on January 21, 2016. Considering the low price, the management declared buy-back offer for Rs.3,500 per share for an aggregate amount of Rs.1,569 crore during February 2016.
Currently, DRL scrip is moving around Rs.3,600 on BSE with market capitalization of around Rs.61,500 crore. At the end of March 2016, DRL promoters were holding 25.6 per cent equity stake and FIIs were holding 38.7 per cent. Mutual funds, banks, corporate bodies, insurance companies and others holding worked out to 11.1 per cent. Individuals were holding only 7.8 per cent and Custodian for ADRs holding were at 16.8 per cent.
The company received US FDA warning letter to its API manufacturing facilities of DRL at Srikakulam, Andhra Pradesh and Miryalaguda, Telangana as well as oncology formulation manufacturing facility at Duvvada, Visakhapatnam, Andhra Pradesh on November 5, 2015. Thereafter, share price movements were impacted and scrip lost investor confidence as the major revenue of over 50 per cent is coming from US markets.
For the challenging year ended March 2016, DRL's consolidated net sales increased by 4.6 per cent only to Rs.15,380 crore from Rs.14,703 crore in the previous year due to lower sales in Europe, Russia & other CIS market. Its global generic net sales increased to Rs.12,922 crore from Rs.12,148 crore and that of pharmaceutical services & active ingredients (PSAI) segment declined by 12.9 per cent to Rs.2,737 crore from Rs.3,143 crore. The sales of proprietary products went by 163 per cent to Rs.266 crore from Rs.101 crore.
Its North American sales contributed 53 per cent to its gross sales and reached at Rs.8,199 crore as against Rs.7,074 crore in the previous year, a growth of 15.9 per cent. Its domestic sales moved up by 11.5 per cent to Rs.2,204 crore from Rs.1,977 crore. However, European sales declined by 1.7 per cent to Rs.1,749 crore from Rs.1,780 crore and that in Russia & other CIS countries nosedived by 19.9 per cent to Rs.1,418 crore from Rs.1,771 crore. Similarly, sales in other markets declined by 13.2 per cent to Rs.1,894 crore due to volatile exchange rates, especially in Venezuela and Russia.
DRL's EBIDTA increased by 11.2 per cent to Rs.4,190 crore during 2015-16 from Rs.3,768 crore in the previous year despite lower other operating income as well as other income. Its service income declined to Rs.147 crore from Rs.169 crore and other operating income to Rs.95 crore from Rs.115 crore. However, license fees income moved up to Rs.77 crore from Rs.37 crore.
The interest burden declined by 23.8 per cent to Rs.82 crore from Rs.108 crore, but depreciation provision went up 27.8 per cent to Rs.971 crore from Rs.760 crore. Taxation provision worked out to Rs.524 crore as against Rs.563 crore. DRL provided Rs.462 crore for foreign exchange loss during 2015-16 which put pressure on bottom line and its net profit declined by 7.9 per cent to Rs.2,151 crore from Rs.2,336 crore. EPS worked out to Rs.126.15 as against Rs.137.18 crore.
As against the equity capital of Rs.85.3 crore, DRL has built up strong reserve & surplus position which stood at Rs.11,616 crore as compared to Rs.9,798 crore in the previous year. The company has reduced its long term borrowing by 25.3 per cent to Rs.1,069 crore from Rs.1,432 crore and its debt equity ratio worked out to 0.26:1 as against 0.39:1 in the previous year. EBDITA margins improved to 27.2 per cent from 25.6 per cent. The company management declared handsome equity dividend of 400 per cent for 2015-16.
DRL has 52 subsidiaries and 3 joint venture companies. It added two new subsidiaries viz., Dr Reddy's Laboratories Japan KK, Japan and Reddy Pharma SAS, France. It also formed a new joint venture company called DRES Energy Pvt Ltd during 2015-16. Its German subsidiary Reddy Specialties GmbH ceased to be a subsidiary as it is merged with Reddy Holding GmbH.
The company's R&D expenditure increased by 6.2 per cent to Rs.1,790 crore from Rs.1,685 crore and worked out to 11 per cent of total revenues. The company filed 50 drug master files (DMFs) and its cumulative number of DMF filings reached at 768. Further, DRL filed 13 ANDAs and 1 NDA with US FDA. Currently, 82 generic filings are pending for approval. With the help of R&D investments, it launched 51 new products in global market including India.
During June 2016, DRL acquired eight ANDAs in the US for US$350 million from Teva Pharmaceutical Industries. The acquired portfolio consists of products that are being divested by Teva as a precondition to its closing of the acquisition of Allergan's generics business. The combined sales of the branded versions of the products in the US is approximately $3.5 billion. Similarly, it acquired over-the-counter (OTC) brands in cough-and-cold, pain and dermatology categories from Ducere Pharma during May 2016.
Though, the financial working of DRL will be under pressure in the first and second quarter of 2016-17 on account of US FDA restrictions and exchange rate movements, the long term prospect looks better with higher investments in R&D, higher approvals, launch of new products and focus on highly regulated markets. |