US FDA accepts Actavis' sNDA for Saphris to treat bipolar I disorder in paediatric patients
The US Food and Drug Administration (US FDA) has accepted for filing Actavis' Supplemental New Drug Application (sNDA) for Saphris (asenapine) for the acute treatment of manic or mixed episodes associated with bipolar I disorder in paediatric patients 10 to 17 years of age. Actavis' sNDA for Saphris has been granted priority review status by the FDA.
"The sNDA filing of Saphris speaks to our commitment to ongoing research and development of our mental health portfolio," said C. David Nicholson, PhD, senior vice president, Actavis Global Brands R&D. "We are pleased that the FDA has accepted this sNDA, marking the first step towards our goal of bringing this important antipsychotic treatment option to paediatric patients."
Actavis expects the Prescription Drug User Fee Act (PDUFA) date to be in Q1 2015. The sNDA submission for asenapine is based on the results of a 3-week monotherapy trial in 403 paediatric patients (10 to 17 years of age), of whom 302 received asenapine. In the trial, asenapine was shown to be statistically superior to placebo in the reductions of both the Young Mania Rating Scale (YMRS) total score and Clinical Global Impression-Bipolar (CGI-BP) score at fixed doses of 2.5 mg, 5 mg and 10 mg twice daily. The most commonly observed adverse reactions (incidence 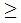 5 per cent and at least twice that for placebo) were somnolence, dizziness, dysgeusia, oral hypoesthesia, oral paresthesia, nausea, increased appetite, fatigue and increased weight.
5 per cent and at least twice that for placebo) were somnolence, dizziness, dysgeusia, oral hypoesthesia, oral paresthesia, nausea, increased appetite, fatigue and increased weight.
SAPHRIS (asenapine) is an atypical antipsychotic that has been prescribed over one million times since it was first approved by the FDA in 2009. It is indicated for the treatment of schizophrenia in adults, and for the acute treatment of manic or mixed episodes associated with bipolar I disorder in adults, as monotherapy or as adjunctive therapy with either lithium or valproate.
Bipolar disorder, which encompasses bipolar I and bipolar II disorders, affects approximately million people in the US Bipolar I disorder, also known as manic-depressive illness, is characterised by unusual shifts in mood, energy, activity levels, and the ability to carry out day-to-day tasks. Patients experience "mood episodes" that manifest as either a manic episode (overexcited, extreme irritability, racing thoughts, and difficulties with sleep) or a depressive episode (extreme sadness, fatigue, or hopelessness), or a combination of both.